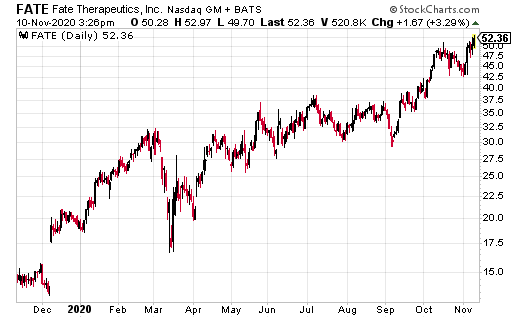
Fate Therapeutics (FATE) is one of the hottest biotech stocks of the year.
Since 2020, FATE ran from a low of $18.80 to a recent high of $48.20, good for a return of 156%, as compared to the iShares Nasdaq Biotech ETF (IBB), which is up 16%.
From here, FATE could see higher highs ahead of its 12 presentations at the 2020 American Society of Hematology (ASH) Annual Meeting and Exposition being held virtually from December 5 to 8, 2020.
Buy and Hold These 3 Dividend Stocks Forever [ad]
The company also released its earnings results over the last few days.
“The clinical data across our iPSC product platform continue to solidify our conviction that multiple doses of iPSC-derived NK cells can be administered off-the-shelf in the outpatient setting, are well-tolerated, and can drive anti-tumor activity, including in combination with monoclonal antibody therapy,” said Scott Wolchko, President and Chief Executive Officer of Fate Therapeutics. “We have now expanded the scope of clinical investigation for FT516 to solid tumors as well as for FT596 to chronic lymphocytic leukemia after observing clinical activity in diffuse large B-cell lymphoma at the first dose level. In addition, we have initiated first-in-human investigation of the first-ever CRISPR-edited, iPSC-derived cell therapy FT538, which incorporates three engineered elements to enhance multiple mechanisms of innate immunity, in acute myeloid leukemia and multiple myeloma.”
In addition, over the last few months, the company entered into an exclusive license agreement with Baylor College of Medicine covering alloimmune defense receptors, a first-in-class approach that renders off-the-shelf allogeneic cell products resistant to host immune rejection.
Preclinical studies published in the journal Nature Biotechnology demonstrate that allogeneic cells engineered with a novel alloimmune defense receptor (ADR) are protected from both T- and NK-cell mediated rejection, and provide proof-of-concept that ADR-expressing allogeneic cell therapies can durably persist in immunocompetent recipients.
FATE recently secured a $3 billion deal with Janssen Biotech, Inc, one of the Janssen Pharmaceutical Companies of Johnson & Johnson to develop four cancer treatments.
The U.S. Food and Drug Administration (FDA) has cleared the Company’s Investigational New Drug (IND) application for FT819, an off-the-shelf allogeneic chimeric antigen receptor (CAR) T-cell therapy targeting CD19+ malignancies. FT819 is the first-ever CAR T-cell therapy derived from a clonal master induced pluripotent stem cell (iPSC) line, and is engineered with several first-of-kind features designed to improve the safety and efficacy of CAR T-cell therapy.
The Company plans to initiate clinical investigation of FT819 for the treatment of patients with relapsed / refractory B-cell malignancies, including chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), and non-Hodgkin lymphoma (NHL).
Ian Cooper’s Personal Position in FATE: None