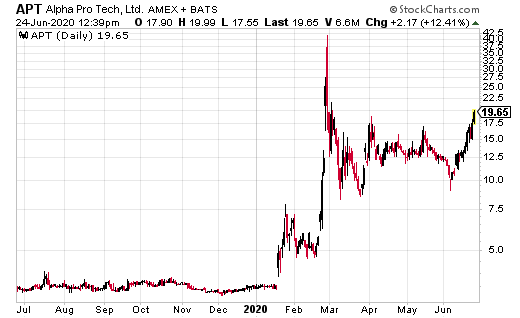
Over the last few weeks, shares of Alpha Pro Tech (APT) have exploded from a low of $9.05 to a recent high of $18.03. All as the number of coronavirus cases soar around the world – again.
“The pandemic is still accelerating,” WHO’s director general Tedros Adhanom Ghebreyesus said. “We know that the pandemic is much more than a health crisis, it is an economic crisis, a social crisis and in many countries a political crisis.
Arizona Gov. Doug Ducey even just noted, “Looking at the last two weeks of data, there is a trend. And the trend is headed in the wrong direction and the actions we’re going to take are intended to change that direction and reverse this trend.”
FREE! Beginner’s Options Guide for Investors Who Have Never Traded Options But Want To. Click Here To Download. [ad]
At the same time, demand for masks is increasing again. California Gov. Gavin Newson just tweeted, “Californians are now REQUIRED to wear face coverings in public spaces.”
And, according to NPR, Newsom added, “Our numbers are going up, not going down. Hospitalization numbers are just starting to creep back up, and I’m very concerned by what we’re seeing. We think the most impactful thing we can do, short of going back to a stay-at-home order, is wearing face coverings when we can’t practice physical distancing.”
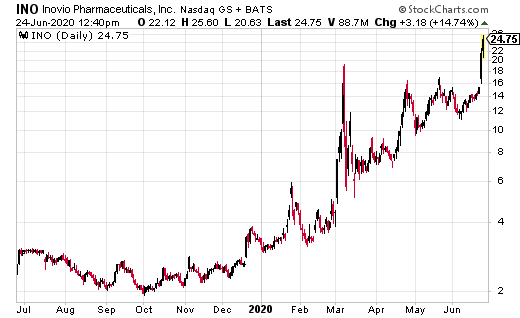
Shares of Inovio Pharmaceuticals (INO) jumped from $12.02 to $22.68 after receiving a $71 million contract from the US Department of Defense to scale up the manufacturing of CELLECTRA 3PSP smart device and the procurement of CELLECTRA 2000 devices, which are used to deliver INO-4800 directly into the skin.
Dr. J. Joseph Kim, INOVIO’s President and CEO, said, “INOVIO is very pleased to receive this significant funding from the U.S. Department of Defense to continue our rapid scale-up capacity for our breakthrough DNA medicines delivery device CELLECTRA.”
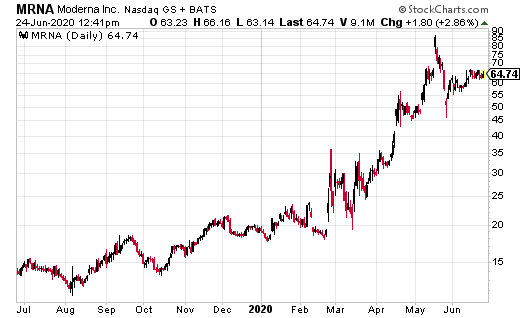
Shares of Moderna Inc. (MRNA) jumped again to $65, and could accelerate higher. All after MRNA has advanced late-stage development of its coronavirus vaccine. Better, it just finalized the Phase 3 study protocol based on feedback from U.S. FDA.
Related: MRNA Stock Price Jumps On Finalizing FDA Phase 3 Study for Coronavirus Vaccine
The randomized, 1:1 placebo-controlled trial is expected to include approximately 30,000 participants enrolled in the U.S. and is expected to be conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). Plus, MRNA has completed manufacture of vaccine required to start the Phase 3 study, and expects dosing in the Phase 3 study to begin in July.
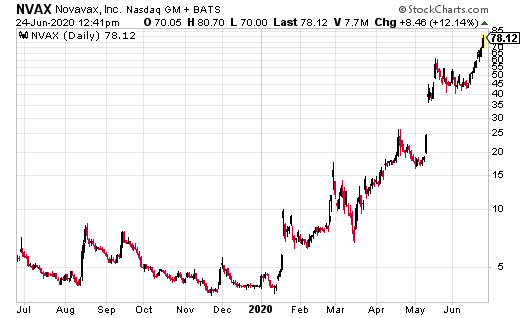
Novavax Inc. (NVAX) jumped from $45 to a high of $78.50 as the number of coronavirus cases explodes higher. The company was just awarded a contract by the U.S. Department of Defense (DoD) for the manufacturing of NVX‑CoV2373, Novavax’ COVID-19 vaccine candidate. NVX‑CoV2373 consists of a stable, prefusion protein antigen made using its proprietary nanoparticle technology and includes Novavax’ proprietary Matrix‑M adjuvant.
“JPEO-CRBND-EB through funding provided by the Defense Health Program, has agreed to fund up to $60 million to support Novavax in its production of several components of the vaccine that will be manufactured in the U.S. The agreement includes a 2020 delivery of 10 million doses of NVX‑CoV2373 for DoD that could be used in Phase 2/3 clinical trials or under an Emergency Use Authorization (EUA) if approved by the U.S. FDA,” according to a NVAX PR.