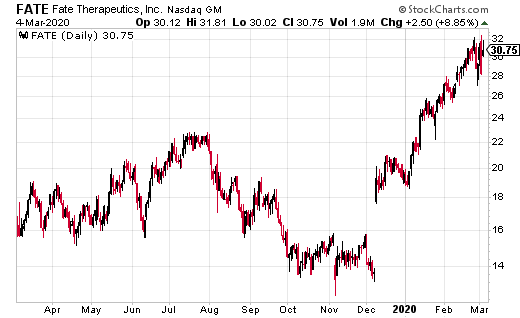
Fate Therapeutics (FATE) just announced earnings March 3, 2020, with a beat on EPS and on revenue. The company’s GAAP EPS loss of 37 cents beat estimates by two cents. Revenue of $2.8 million beat estimates by $1.1 million, and was up 68.7% year over year.
Related: Fate Therapeutics Reports Fourth Quarter 2019 Financial Results
“In 2019, we made tremendous progress in pioneering the clinical development of off-the-shelf, iPSC-derived cancer immunotherapy,” said President and CEO Scott Wolchko.
“Our FT500 program demonstrated that multiple doses of iPSC-derived NK cells can be delivered off-the-shelf to a patient in a safe manner without patient matching. Additionally, our FT516 program provided initial clinical evidence that engineered iPSC-derived NK cells may confer anti-tumor activity and deliver clinically meaningful benefit to patients. We also showed the unmatched scalability of our proprietary iPSC product platform, having manufactured hundreds of cryopreserved, infusion-ready doses of our iPSC-derived NK cell product candidates at a low cost per dose in our new GMP manufacturing facility.”
Further Reading: How NK May Offer a Better Cancer Treatment Than CAR-T
Going forward, the company will soon present additional clinical data from its FT500 and FT516 programs, as well as data from its FT596, and an iPSC-derived CAR NK cell product candidate for B-cell malignancies. At the moment, the company is pushing forward with the following:
FT500 Phase I study Results Encouraging
To date, the company has seen encouraging safety, tolerability and immunogenicity data from its FT500 Phase 1 study. In Dec. 2020, the company reported initial clinical data from the dose-escalation stage of the FT500 Phase 1 study for the treatment of advanced solid. The company found no adverse events or serious adverse events, and no incidents of cytokine release syndrome, neurotoxicity, or graft-versus-host disease.
FT516 Showed No Serious Adverse Events
In December, the Company announced that the first two patients were treated with FT516, the Company’s off-the-shelf NK cell cancer immunotherapy derived from a clonal master iPSC line engineered to express a novel high-affinity, non-cleavable CD16 (hnCD16) Fc receptor, says the company. There were no FT516-related adverse events or serious adverse events and no incidents of cytokine release syndrome, neurotoxicity, or graft-versus-host disease.
FT516 Clinical Investigation Expanded to Solid Tumors
In early 2020, Fate Therapeutics announced the U.S. FDA allowed Fate’s second IND application for FT516, which allows for the clinical investigation of FT516 in combination with PDL-1, PD1-, EGFR- and HER2-targeting monoclonal antibody (mAb) therapies across a broad range of solid tumors.
FT596 Patient Enrollment Initiated for Advanced B-cell Malignancies
The Company is conducting an open-label Phase 1 clinical trial of FT596, the Company’s first off-the-shelf, iPSC-derived chimeric antigen receptor (CAR) NK cell cancer immunotherapy and the first cellular immunotherapy engineered with three active anti-tumor modalities, to be cleared for clinical investigation by the FDA.
Ian Cooper’s Personal Position in FATE: None





