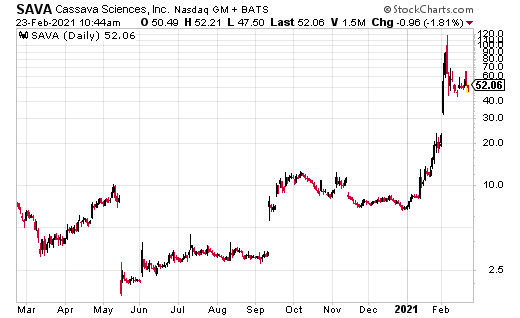
Cassave Sciences Inc. (SAVA) has been one of the most explosive biotech stocks of 2021.
Since January, SAVA has run to the current price of $63 from a low of $8.15. It’s up another $7.22 on the day after announcing a positive end of Phase 2 meeting with the U.S. Federal Drug Administration (FDA). SAVA has also outlined plans for its pivotal program for Simufilam for Alzheimer’s disease.
“Official EOP2 meeting minutes indicate FDA and Cassava Sciences agree on key elements of a pivotal Phase 3 clinical program in support of a New Drug Application (NDA) filing for simufilam in Alzheimer’s disease. Agreements reached during the EOP2 meeting show a clear path forward for advancing simufilam into Phase 3 studies in the second half of 2021,” according to the company’s latest press release.
CEO, Remi Barbier noted, “We are excited to finally advance simufilam into pivotal Phase 3 clinical studies in people with Alzheimer’s disease. We believe the underlying science is solid, the drug appears safe and the clinical roadmap makes sense. We’ve crossed the Rubicon.”
In addition, Cassava Sciences’ second Phase 3 study is designed to evaluate symptomatic improvement in Alzheimer’s disease. The goal is to demonstrate improved cognition and health function in subjects treated with simufilam compared to placebo.
Details of the second Phase 3 study include:
- Approximately 600 subjects with mild-to-moderate Alzheimer’s disease to be enrolled.
- Subjects to be randomized (1:1) to simufilam 100 mg or placebo BID.
- Subjects to be treated for 9 to 12 months.
- The co-primary efficacy endpoints are ADAS-Cog, a cognitive scale, and ADCS-ADL, a functional scale; both are widely used clinical tools in trials of Alzheimer’s disease.
- A secondary efficacy endpoint is iADRS, a widely used clinical tool in trials of Alzheimer’s disease that combines cognitive and functional scores from ADAS-Cog & ADCS-ADL.
- Other secondary endpoints include biomarkers of disease and NPI, a clinical tool that assesses the presence and severity of dementia-related behavior.
- The Company plans to initiate the second pivotal Phase 3 study Q4 2021.
At the time of this writing, Ian Cooper did not hold a position in Cassava Sciences Inc. (SAVA).