TransMedics (TMDX) is a very interesting company that is reimagining traditional transplant therapy for end-stage organ failure and several diseases. Its Organ Care System (OCS) is a novel concept that works better than current standards as its replicates natural living and functioning of an organ outside of the body.
The technology keeps donor organs, such as lungs, hearts, and livers in pristine condition while preserving them for transplant operations at a much higher rate. These three organ transplant segments comprise a very large market and bode well for the company’s prospects for multi-year growth.
The medical device company is actively engaged with the Food and Drug Administration (FDA) to get approval for its OCS products surrounding the three aforementioned organs.
Earlier this month, TMDX announced the FDA issued a favorable 14-0 vote in support of approving its OCS Liver System. This was a key milestone as the company moves towards potential FDA approval for patients with end-stage liver failure.
This was the latest nod of approval from the agency. In April, the company announced an FDA advisory panel had granted the OCS Heart System a favorable review by a vote of 12 to 5, with one abstention, that indicated the benefits of the technology outweigh its risks. The panel also voted 10 to 6, with two members abstaining, that there is reasonable assurance that the OCS Heart System is effective.
There should be news forthcoming about TransMedics’ OCS Lung System and the company has stated they are confident in their ability to have all three of their OCS products approved and commercially available in the second half of this year.
As with any medical device company, there are risks to getting final clearance for all three products, but so far, things are setting up nicely for TMDX to have long-term success. The global transplantation market is expected to reach $26.5 billion by 2028, as organ failure has resulted in high demand for transplantation procedures.
The company has lost money since becoming publicly traded in May 2019. It is currently expected to post a loss of $0.28 per share for its current quarter on revenue just under $8 million.
For 2021, the company is expected to lose $1.07 per share on revenue around $40 million. For 2022, these numbers improve to a loss of $0.57 per share on sales above $80 million.
Only five analysts cover the stock, and these numbers could come in better—or worse—than expected. Two of the analysts have given TMDX strong buy ratings, with another rating it a buy. The other two analysts have a hold rating on the stock.
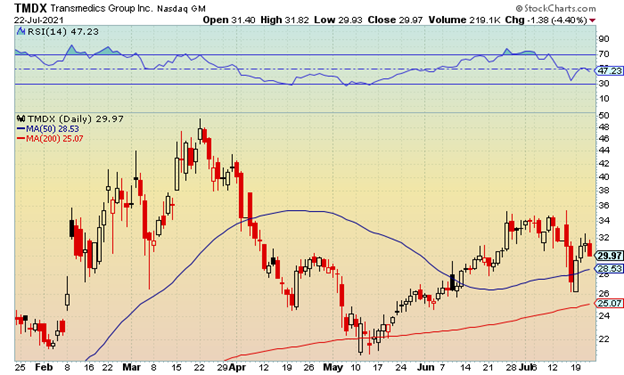
The current chart shows key resistance at the $34 level and near-term support just above the $26 area.
Given the current fundamental analysis, shares remain a risk until TransMedics can start showing a profit. However, on a technical setup, bullish traders can target continued closes above the $34 level to get long. Bearish traders can target a drop below $26 to get short the stock.
Near-term options are also available to trade on the stock, with the furthest going out into January 2022. The calls and puts have hefty premiums given the risk/ reward of the FDA approval process. With shares near the $30 level, the TMDX January 30 calls and puts are trading in the $5-6 range.
To make a triple-digit return on the calls, shares will need to make a run at $40 by January 21, 2022. If shares fall towards $20, the put options would likely double. However, if you are on the wrong side of the trade, the call or put options could expire worthless if these levels are achieved.
The stock will need to be above $35 for the call options to likely breakeven, or below $25 for the put options. At these levels, the calls or puts would be $5 in the money and where the current premiums are for both options.